 The Management Board of Scope Fluidics SA has decided to extend the work on a panel for detecting viral respiratory tract infections by developing an ultra-fast test for detecting the SARS-CoV-2 virus, which causes the Covid-19 disease. The test is expected to be ready for validation as early as autumn/winter 2020.
The Management Board of Scope Fluidics SA has decided to extend the work on a panel for detecting viral respiratory tract infections by developing an ultra-fast test for detecting the SARS-CoV-2 virus, which causes the Covid-19 disease. The test is expected to be ready for validation as early as autumn/winter 2020.
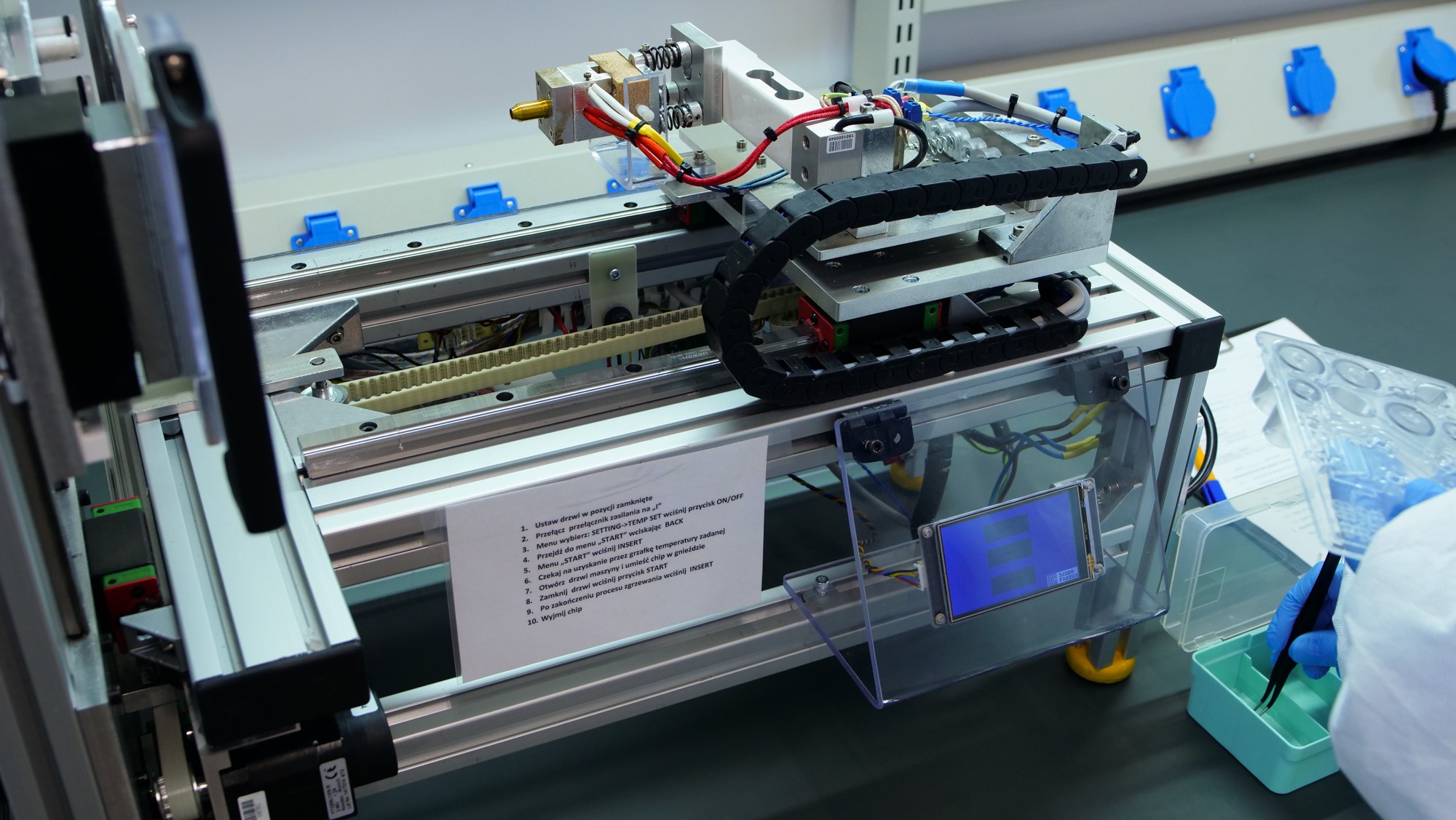
PCR|ONE is a universal bacterial and virus pathogen detection system developed by Scope Fluidics. Samples are tested automatically with results available within 15 minutes. As the development project is nearing completion, new tests, including virus detection tests, may be available shortly along with its MRSA panel.
– The PCR|ONE system is a perfect solution for applications such as mass testing, for instance at airports, railway stations, and other crowded venues where fast sample-to-result is critical. What is key in such applications is the test can be carried out at the sampling location in order to avoid the logistics necessary to send the sample to a lab. PCR|ONE offers the great advantage of speed, as the result is available within minutes, combined with multiplexity, where detection of other infections with similar clinical symptoms may be added to the panel. The Covid-19 pandemic is an enormous challenge and a clear indication that fast diagnostics is urgently needed, – said Professor Piotr Garstecki, Co-Founder and CEO of Scope Fluidics.
The development of a panel for detecting the SARS-CoV-2 virus, which causes the Covid-19 disease, will be undertaken as part of the work on extending the portfolio of the PCR|ONE diagnostic tests and will include optimisation of the sample cleaning procedure for extraction of genetic material from throat swabs, transcription of the material, and detection of infection markers. The system will eventually be ready for simultaneous diagnostics of several pathogens, such as SARS-CoV-2, influenza A and B, RSV. The panel could also be extended to include other pathogens on the WHO alert list, such as MERS-CoV, SARS-CoV, swine flu, measles, rubella.
The work will be financed with the company’s own resources. The company may also raise funding for certification and start of production of SARS-CoV-2 tests for instance from the European Union funds supporting high technology and health care.
The development and validation of such systems takes time and effort. Luckily, we are approaching completion of the work necessary to certify the first panel for detecting MRSA in mid-2020. This phase opens the door to the development of a portfolio of diagnostic panels, – said Professor Piotr Garstecki.
Recent consultations confirm that the performance parameters of the PCR|ONE system optimally respond to the need for rapid, sensitive and reliable tests for detecting the SARS-CoV-2 virus at the earliest possible stage of infection. The functionality of the PCR|ONE system also addresses the trend in the rapidly growing market for molecular diagnostics in the point-of-care format by combining the speed of operation, the range of detected genetic markers and a low cost of panel production.
It typically takes from one to three days to get test results while all contacts with those who are infected augment the spread of the virus. To cut the waiting time, point-of-care tests check the material right at the sampling location. This is the philosophy behind the PCR|ONE system.
We are glad to see an emergence of point-of-care genetic tests for detection of the SARS-CoV-2 virus. We are a start-up but we are making best efforts to promptly join the fight with the pandemic based on the speed and multiplexity of the PCR|ONE system, – said Professor Piotr Garstecki.